CoolSculpting®, Reimagined
Over 75 million people in the United States would consider noninvasive fat reduction treatment.1,* And with over 13 million treatments worldwide,2 patients consistently turn to CoolSculpting to help them reduce stubborn fat. For over 10 years, CoolSculpting has led the body contouring industry and is the #1 nonsurgical fat reduction treatment used most by doctors† —and most recognized by patients.3
We’ve created CoolSculpting® Elite to help take your practice further. From the slickest touchscreen interface to an increased chiller capacity to support dual applicator use, we thoughtfully considered every detail.
* Survey of consumers ages 18 to 75 years with a BMI of 18.5 to 34.9 and an annual household income of $35,000 or more (n=11121). US population, age, and income data from 2018 US census projections.
† CoolSculpting® is the treatment doctors use most for nonsurgical fat reduction.
Contact Us
Internal Error
CoolSculpting® and CoolSculpting® Elite Indications
CoolSculpting® and CoolSculpting® Elite are FDA-cleared for the treatment of visible fat bulges in the thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm in patients with a Body Mass Index (BMI) of ≤ 30 and in submental and submandibular areas in patients with a BMI of ≤ 46.2. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.
CoolSculpting® and CoolSculpting® Elite Important Safety Information
CoolSculpting® and CoolSculpting® Elite are contraindicated in patients with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Ask your patient about any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. Paradoxical hyperplasia (visibly enlarged tissue volume in the treated area) may develop 2 to 5 months after treatment, will not resolve on its own, and may require surgical intervention for correction.
As with any medical procedure, a consultation should be done by a licensed healthcare professional to determine if the patient is a candidate for treatment. For a complete list of Contraindications, Warnings, Precautions, and Potential Side Effects, consult the CoolSculpting® System User Manual. and the CoolSculpting® Elite System User Manual. Treatment applications that deviate from the guidelines are not recommended.
CoolTone® Indications
The CoolTone® device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone® is also indicated for strengthening, toning, and firming of buttocks and thighs.
CoolTone® Important Safety Information
CoolTone® treatment is contraindicated in placing the active applicator over metal, electrical, or electronic implants/devices in the treatment area like cardiac pacemakers, cochlear implants, intrathecal pumps, implanted defibrillators, implanted neurostimulators, drug pumps, or hearing aids.
CoolTone® is also contraindicated in placing the active applicator over menstruating uterus, over areas of the skin that lack normal sensation, and in patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure, pulmonary insufficiency, or pregnancy.
CoolTone® should be used with caution in patients with Graves’ disease, active bleeding disorders, or seizure disorders.
Women who are close to menstruation may find that it comes sooner, or cramping is increased or intensified with CoolTone® treatments, therefore, it is recommended to not undergo treatment during this time of the month.
CoolTone® should not be used in the heart or head areas, areas of growth plate, over the carotid sinus nerves, or over the neck or mouth. CoolTone® should not be applied over swollen, infected, inflamed areas or skin eruptions. Caution should be used for patients with suspected or diagnosed heart problems.
Ensure that persons with pacemakers are not present in vicinity of the device during treatment.
Common adverse effects may include, but may not be limited to muscular pain, temporary muscle spasm, temporary joint or tendon pain, and local erythema or skin redness.
Consult the CoolTone® User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
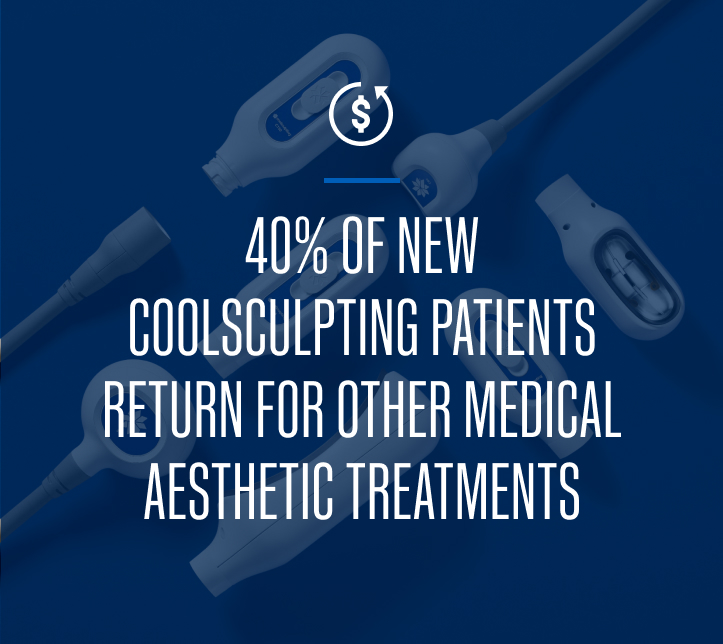
References: 1. Data on file, Allergan; Aesthetic Foundational Sizing & Allergan Category Opportunity. 2. Data on file, Allergan, September 8, 2022; Body Contouring Device Health Dashboard 13+ million Cycles. 3. Data on file, Allergan; CoolSculpting Consumer Awareness Q4 2017. 4. Data on File; Allergan, December 2016, Survey Summary.