
Real patients, real transformations.
CoolTone delivers real muscle toning results to patients and can help them achieve their body goals.
SEE RESULTS
Real opportunity for your practice.
Capitalize on growth potential by pairing CoolTone with CoolSculpting.
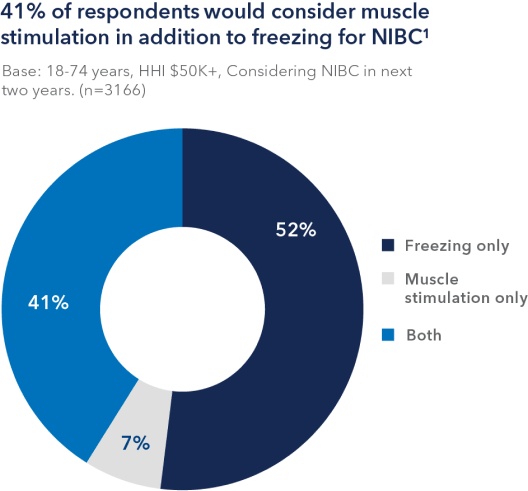
Over 4,000,000 people are willing to consider noninvasive body contouring within the next year. Body contouring is one of the top drivers of aesthetic market growth. With this demand, now is a great time to consider adding CoolTone to your practice.
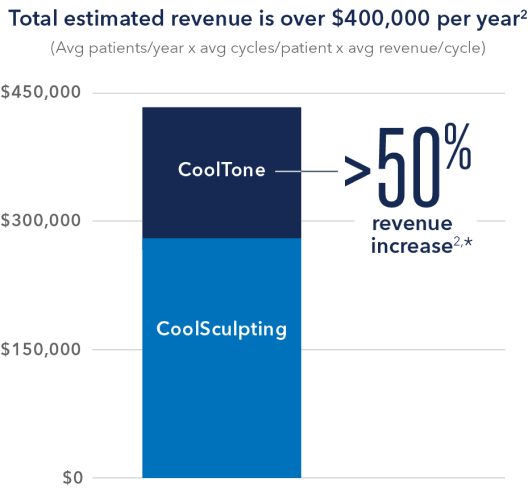
Adding CoolTone to your practice can lead to more revenue.
With an increase in demand for muscle toning, adding CoolTone could result in up to 50% more body contouring revenue for your practice.
Note: The safety and efficacy of CoolSculpting® and CoolTone® in combination have not been evaluated by the FDA.
Trusted Allergan Aesthetics Partnership.
Choosing CoolTone means you'll have access to ongoing support, including the confidence inherent in a trusted Allergan partnership and unprecedented benefits for you and your patients.


Physician Loyalty Programs

Patient Loyalty Program
CoolTone® Indications
The CoolTone® device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone® is also indicated for strengthening, toning, and firming of buttocks and thighs.
CoolTone® Important Safety Information
CoolTone® treatment is contraindicated in placing the active applicator over metal, electrical, or electronic implants/devices in the treatment area like cardiac pacemakers, cochlear implants, intrathecal pumps, implanted defibrillators, implanted neurostimulators, drug pumps, or hearing aids.
CoolTone® is also contraindicated in placing the active applicator over menstruating uterus, over areas of the skin that lack normal sensation, and in patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure, pulmonary insufficiency, or pregnancy.
CoolTone® should be used with caution in patients with Graves’ disease, active bleeding disorders, or seizure disorders.
Women who are close to menstruation may find that it comes sooner, or cramping is increased or intensified with CoolTone® treatments, therefore, it is recommended to not undergo treatment during this time of the month.
CoolTone® should not be used in the heart or head areas, areas of growth plate, over the carotid sinus nerves, or over the neck or mouth. CoolTone® should not be applied over swollen, infected, inflamed areas or skin eruptions. Caution should be used for patients with suspected or diagnosed heart problems.
Ensure that persons with pacemakers are not present in vicinity of the device during treatment.
Common adverse effects may include, but may not be limited to muscular pain, temporary muscle spasm, temporary joint or tendon pain, and local erythema or skin redness.
Consult the CoolTone® User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
References:
1. Source: Allergan Aesthetics 2020 Market Sizing. Question S29a: “You indicated you are willing to consider receiving non-surgical body contouring in the future. There are multiple ways to contour the body. How likely are you to consider each of the following ways to contour your body?
2. Data on file, Allergan, December 2019; CoolSculpting® and CoolTone® Average Revenue Growth.
*Estimated figure is a percentage increase (≈48%) based on an office’s total CoolSculpting revenue of ≈$260,000 and an office’s total CoolTone revenue of ≈$140,000.



