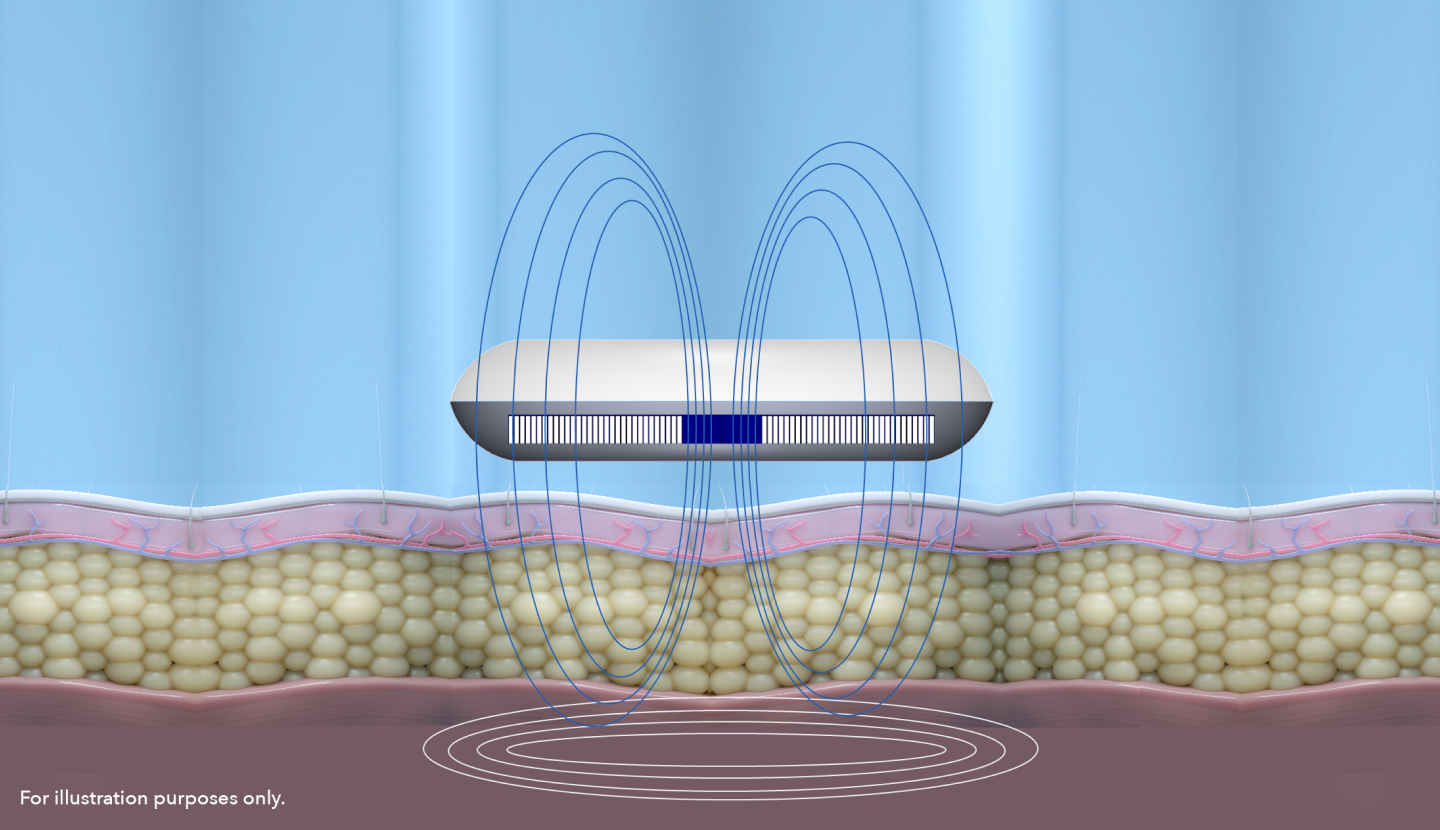
The Science of Muscle Stimulation.
Electromagnetic Muscle Stimulation uses three parameters to modulate contractions: intensity, frequency and pulse width.

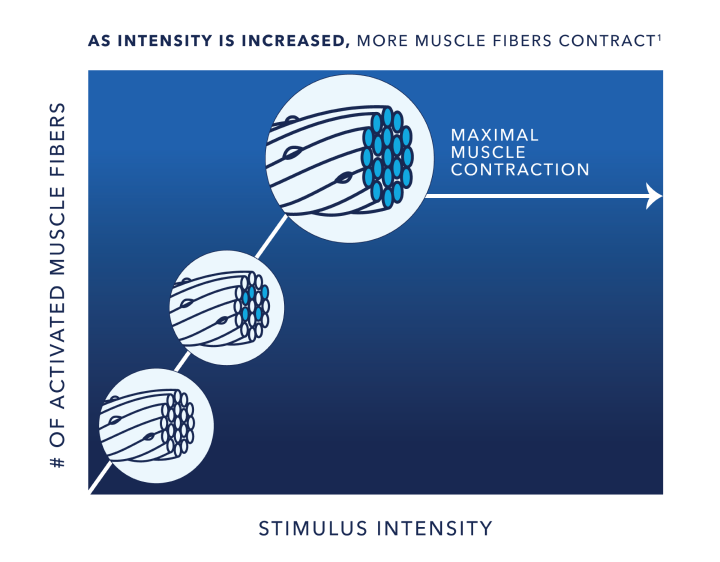
Intensity:
Muscle Excitation
The more muscle fibers that are activated, the more powerful the contraction.
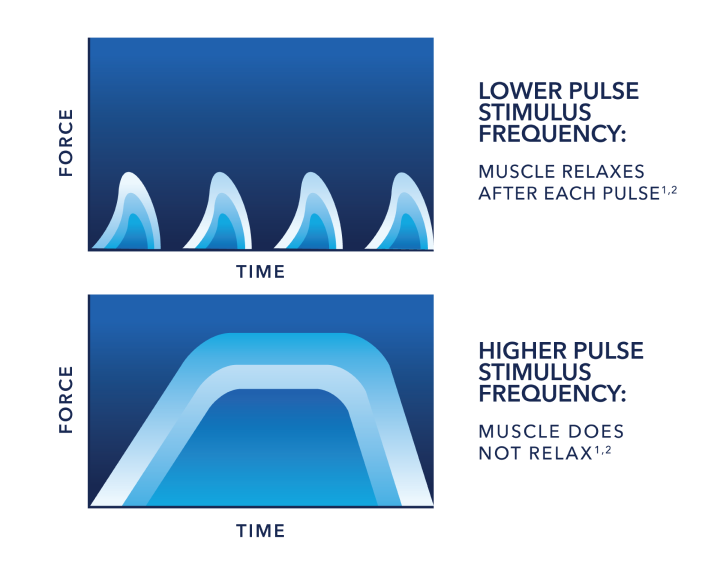
Frequency:
Muscle Response
The higher pulse frequency keeps muscle fibers from relaxing, meaning the force increases and muscles work harder.
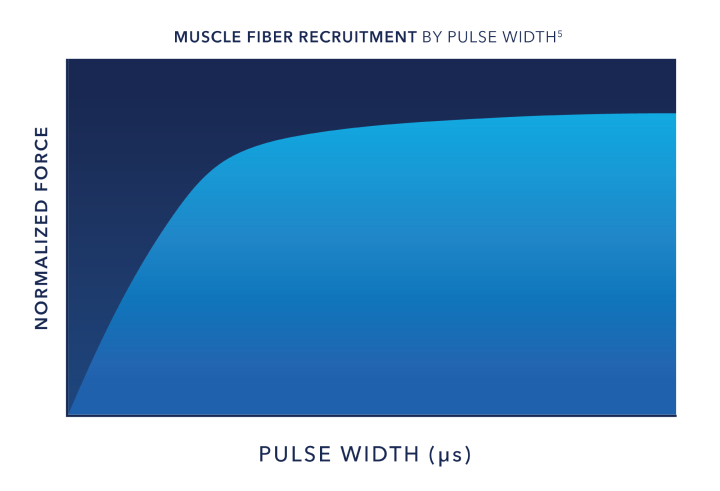
Pulse Width:
Muscle Recruitment
The longer the pulse width, the deeper and more powerful the muscle contraction. 2,3,4
CoolTone® Indications
The CoolTone® device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone® is also indicated for strengthening, toning, and firming of buttocks and thighs.
CoolTone® Important Safety Information
CoolTone® treatment is contraindicated in placing the active applicator over metal, electrical, or electronic implants/devices in the treatment area like cardiac pacemakers, cochlear implants, intrathecal pumps, implanted defibrillators, implanted neurostimulators, drug pumps, or hearing aids.
CoolTone® is also contraindicated in placing the active applicator over menstruating uterus, over areas of the skin that lack normal sensation, and in patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure, pulmonary insufficiency, or pregnancy.
CoolTone® should be used with caution in patients with Graves’ disease, active bleeding disorders, or seizure disorders.
Women who are close to menstruation may find that it comes sooner, or cramping is increased or intensified with CoolTone® treatments, therefore, it is recommended to not undergo treatment during this time of the month.
CoolTone® should not be used in the heart or head areas, areas of growth plate, over the carotid sinus nerves, or over the neck or mouth. CoolTone® should not be applied over swollen, infected, inflamed areas or skin eruptions. Caution should be used for patients with suspected or diagnosed heart problems.
Ensure that persons with pacemakers are not present in vicinity of the device during treatment.
Common adverse effects may include, but may not be limited to muscular pain, temporary muscle spasm, temporary joint or tendon pain, and local erythema or skin redness.
Consult the CoolTone® User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
References:
1. Marieb EN, Hoehn KN. Muscles and muscle tissue. In: Marieb E, Hoehn K, eds.Human Anatomy & Physiology. 11th ed. New York, NY: Pearson; 2019:299-304.
2. Betts JG, Desaix P, Johnson E, et al. Muscle tissues. In: Betts J, Desaix P, Johnson E,et al, eds. Anatomy and Physiology. Houston, TX: OpenStax College; 2013. https://opentextbc.ca/anatomyandphysiology/chapter/10-1-overview-of-muscle-tissues. Accessed November 20, 2020.
3. Man WD, Moxham J, Polkey MI. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J. 2004;24(5):846-860.
4. Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85(2):201-215.
5. Crago PE, Peckham PH, Thrope GB. Modulation of muscle force by recruitment during intramuscular stimulation. IEEE Trans Biomed Eng. 1980;27(12):679-684.